Change Location
- Patients and Visitors
Patients and Visitors
Get personalized, coordinated care across Northern California through the Sutter network.
View Patient ResourcesMy Health Online
Make appointments, message your doctor and more through our patient portal.
Enroll NowBilling and Insurance
- Treatments and Services
Treatments and Services
Access award-winning, comprehensive primary and specialty care for your whole family.
View All ServicesFeatured Services
- Research and Education
Research and Education
Our contributions to medical research and education lead to better healthcare outcomes.
Research at SutterGraduate Medical Education
Join our robust training programs led by nationally known healthcare leaders.
Explore Opportunities - About Sutter
About Us
Explore the ways we provide innovative, compassionate care through our network.
Learn About Sutter HealthWork at Sutter Health
Shape the future of healthcare and build your career within our diverse teams.
Find JobsCommunity
- BOOK APPOINTMENT
Patient Resources
Billing and Insurance
Patients and Visitors
Get personalized, coordinated care across Northern California through the Sutter network.
View Patient ResourcesMy Health Online
Make appointments, message your doctor and more through our patient portal.
Enroll Now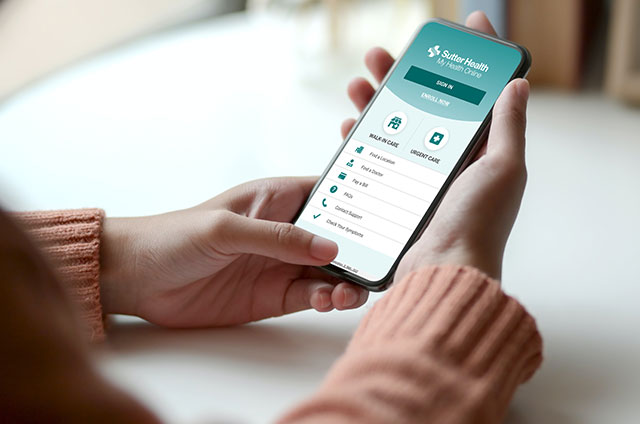
My Health Online
Make appointments, message your doctor and more through our patient portal.
Enroll NowFeatured Services
Treatments and Services
Access award-winning, comprehensive primary and specialty care for your whole family.
View All ServicesResearch and Education
Our contributions to medical research and education lead to better healthcare outcomes.
Research at SutterGraduate Medical Education
Join our robust training programs led by nationally known healthcare leaders.
Explore Opportunities
Graduate Medical Education
Join our robust training programs led by nationally known healthcare leaders.
Explore OpportunitiesAbout Sutter Health
Community
About Us
Explore the ways we provide innovative, compassionate care through our network.
Learn About Sutter HealthWork at Sutter Health
Shape the future of healthcare and build your career within our diverse teams.
Find Jobs
Work at Sutter Health
Shape the future of healthcare and build your career within our diverse teams.
Find JobsCheck-ups, screenings and sick visits for adults and children.
Expertise and advanced technologies in all areas of medicine.
For serious accidents, injuries and conditions that require immediate medical care.
After-hours, weekend and holiday services.
Convenient walk-in care clinics for your non-urgent health needs.
Cookie Policy
We use cookies to give you the best possible user experience. By clicking 'Accept Cookies', you agree to the use of cookies. Privacy Policy Cookie Preferences






